Betamethasone cream has emerged as a highly effective first-line treatment for phimosis, offering a non-surgical solution that can successfully resolve foreskin tightness in approximately 65-95% of cases. This potent topical corticosteroid works by reducing inflammation and increasing tissue elasticity, making it particularly valuable for both paediatric and adult patients experiencing difficulty with foreskin retraction. Unlike invasive surgical procedures, betamethasone treatment provides a conservative approach that preserves the natural anatomy while addressing the underlying pathophysiology of phimotic foreskin tissue. The cream’s anti-inflammatory properties help break down the fibrotic tissue that often contributes to foreskin tightness, whilst simultaneously promoting healthy tissue remodelling.
Understanding betamethasone dipropionate 0.05% cream for phimosis treatment
Betamethasone dipropionate 0.05% cream represents one of the most researched and clinically validated topical treatments for phimosis management. This high-potency corticosteroid belongs to the class IV category of topical steroids, making it significantly more effective than lower-potency alternatives for treating the thick, resistant tissue commonly found in phimotic foreskins. The cream’s formulation allows for optimal penetration into the preputial tissue, where it can exert its therapeutic effects most effectively.
The pharmaceutical composition of betamethasone dipropionate includes specific excipients that enhance skin penetration and provide sustained drug delivery to the target tissues. This formulation ensures that the active ingredient remains in contact with the foreskin for extended periods, maximising therapeutic benefit. Clinical studies have consistently demonstrated that betamethasone 0.05% cream achieves superior outcomes compared to lower-concentration formulations, with success rates ranging from 67% to 95% depending on patient age and phimosis severity.
Topical corticosteroid mechanism of action in foreskin tissue
The therapeutic mechanism of betamethasone in phimosis treatment involves multiple interconnected pathways that work synergistically to restore normal foreskin function. When applied to phimotic tissue, betamethasone penetrates the epidermis and dermis, where it binds to specific glucocorticoid receptors within skin cells. This binding initiates a cascade of anti-inflammatory responses, including the suppression of pro-inflammatory cytokines such as interleukin-1, tumour necrosis factor-alpha, and various chemokines that contribute to tissue fibrosis and scarring.
Beyond its anti-inflammatory effects, betamethasone significantly alters collagen metabolism within the foreskin tissue. The medication reduces excessive collagen synthesis whilst promoting the breakdown of existing fibrous tissue through enhanced collagenase activity. This dual action helps restore the normal elasticity and pliability of the foreskin, allowing for gradual stretching and eventual normal retraction. The remodelling process typically begins within the first two weeks of treatment , though visible improvements may take longer to become apparent.
Betamethasone potency classification and anti-inflammatory properties
Betamethasone dipropionate occupies the Class IV (high potency) category within the topical corticosteroid classification system, making it approximately 25 times more potent than hydrocortisone. This high potency proves essential for treating phimosis because the foreskin tissue often exhibits significant fibrosis and scarring that requires robust anti-inflammatory intervention. The medication’s vasoconstrictor assay demonstrates consistent high-potency characteristics, ensuring reliable therapeutic effects across different patient populations.
The anti-inflammatory properties of betamethasone extend beyond simple inflammation reduction to include comprehensive tissue healing mechanisms. The medication inhibits phospholipase A2, reducing the production of inflammatory mediators like prostaglandins and leukotrienes. Additionally, betamethasone stabilises lysosomal membranes, preventing the release of destructive enzymes that can perpetuate tissue damage and scarring. These multifaceted anti-inflammatory effects make betamethasone particularly suitable for addressing the complex pathophysiology of phimotic foreskin tissue.
Clinical efficacy studies: ashfield and nicholls protocol results
The landmark study by Ashfield and Nicholls established the gold standard protocol for betamethasone treatment in phimosis, demonstrating remarkable success rates of 76% in their cohort of 194 boys. Their research protocol involved twice-daily application of betamethasone 0.05% cream for four weeks, combined with gentle retraction exercises performed by parents. The study’s significance lies not only in its impressive success rates but also in its demonstration that topical steroid therapy could effectively replace circumcision in the majority of phimosis cases.
Subsequent clinical studies have consistently validated these findings, with success rates ranging from 65% to 95% depending on patient selection criteria and treatment duration. A comprehensive meta-analysis of 12 randomised controlled trials involving over 1,400 patients confirmed that betamethasone cream achieves statistically significant improvements in foreskin retractability compared to placebo treatments. These studies collectively demonstrate that betamethasone therapy represents a highly effective, evidence-based treatment option that should be considered as first-line therapy for most phimosis cases.
Contraindications and patient selection criteria
Whilst betamethasone cream demonstrates excellent safety profiles in most patients, certain contraindications and precautions must be carefully considered before initiating treatment. Absolute contraindications include known hypersensitivity to betamethasone or any cream excipients, active bacterial or fungal infections of the genital area, and viral infections such as herpes simplex in the treatment area. Patients with compromised immune systems require careful monitoring, as topical corticosteroids can potentially suppress local immune responses.
Patient selection criteria should prioritise individuals with true phimosis rather than normal developmental foreskin adherence. Children under two years of age rarely require intervention , as physiological phimosis represents a normal developmental stage that typically resolves spontaneously. Healthcare providers must distinguish between pathological phimosis requiring treatment and normal developmental variations that simply require patience and reassurance. Ideal candidates for betamethasone therapy include boys over five years of age with persistent foreskin tightness and adults with acquired phimosis secondary to infection or inflammation.
Proper application technique and dosage protocol
Successful betamethasone treatment for phimosis depends heavily on proper application technique and adherence to established dosage protocols. The standard treatment regimen involves applying a thin layer of betamethasone 0.05% cream to the affected foreskin area twice daily, typically morning and evening, for a period of 4-8 weeks. This frequency ensures optimal tissue penetration and sustained therapeutic levels whilst minimising the risk of systemic absorption. The timing of applications should be consistent to maintain steady drug levels in the target tissues.
The quantity of cream required for each application remains relatively small, typically equivalent to a rice grain-sized amount for paediatric patients and slightly more for adults. Excessive cream application does not enhance therapeutic efficacy and may increase the risk of systemic absorption, particularly in young children. The key principle involves achieving adequate tissue coverage without oversaturation , ensuring that the medication can penetrate effectively whilst avoiding wasteful excess that may cause unnecessary side effects or economic burden.
Pre-application hygiene and foreskin preparation methods
Optimal hygiene practices before betamethasone application significantly enhance treatment efficacy and reduce the risk of secondary infections. Patients should gently cleanse the genital area with warm water and mild, fragrance-free soap, ensuring complete removal of any smegma, urine residue, or previous cream applications. The area should be thoroughly dried with a clean, soft towel using gentle patting motions rather than rubbing, which could cause irritation or micro-trauma to the sensitive foreskin tissue.
Temperature considerations play an important role in preparation, as warm water helps relax the foreskin tissue and facilitates easier cream application. Some practitioners recommend brief warm water soaks for 5-10 minutes before cream application, particularly in cases of severe phimosis where even minimal manipulation proves difficult. This preparatory warming can significantly improve patient comfort during the application process whilst potentially enhancing the cream’s penetration into the tissue layers.
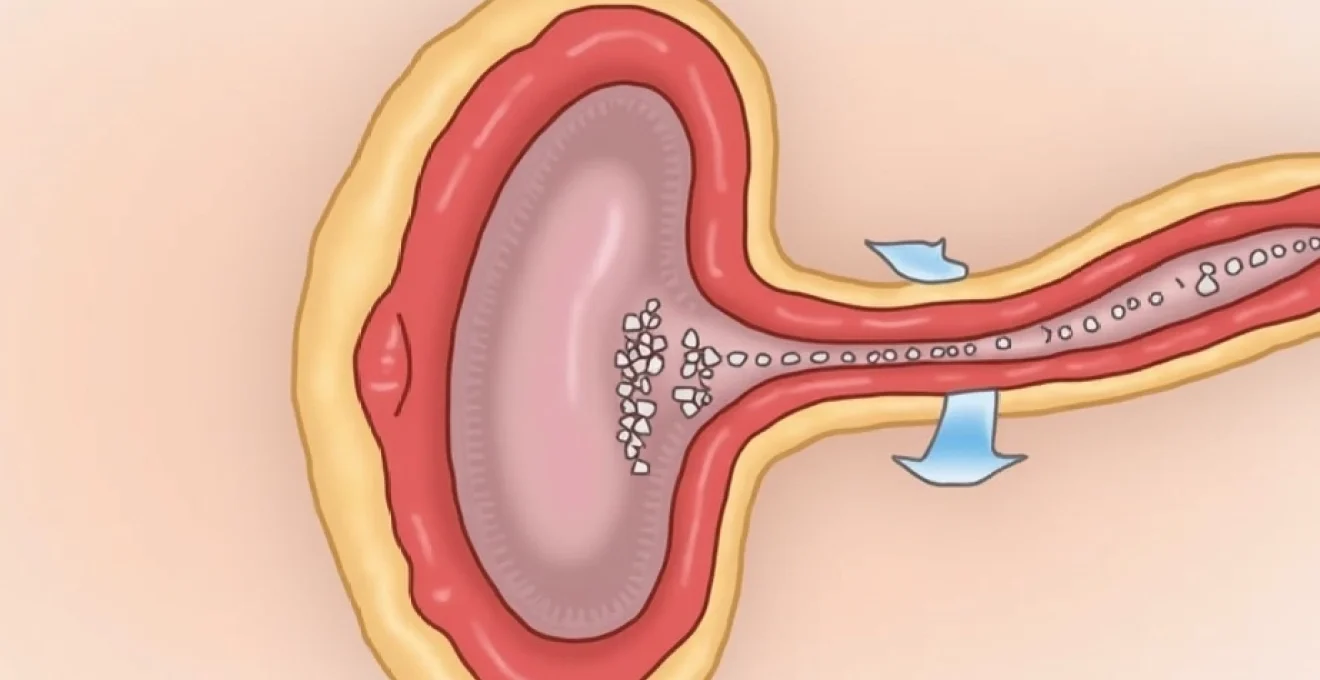
Precise cream application to preputial ring and glans corona
The anatomical precision of betamethasone application proves crucial for achieving optimal therapeutic outcomes in phimosis treatment. The primary target area encompasses the preputial ring, which represents the tightest portion of the foreskin that prevents normal retraction. This area requires careful, gentle massage with the cream, ensuring complete coverage of the circumferential band that creates the mechanical obstruction. The application should extend from the corona of the glans proximally along the foreskin for approximately 1-2 centimetres.
Proper technique involves using clean fingertips to apply gentle circular motions, working the cream into the tissue without forcing retraction beyond the point of comfort. The massage action itself contributes to the therapeutic effect by promoting blood circulation and facilitating drug penetration into deeper tissue layers. Patients must avoid aggressive manipulation that could cause micro-tears or inflammation , as these complications can actually worsen the phimotic condition and delay healing. The application process should feel comfortable and should be stopped immediately if pain or significant resistance occurs.
Gentle retraction exercises during treatment phase
Concurrent gentle retraction exercises form an integral component of successful betamethasone therapy for phimosis, working synergistically with the medication to restore normal foreskin function. These exercises should be performed immediately after cream application, whilst the tissue remains supple and the medication is actively penetrating. The technique involves applying gentle, steady traction to gradually stretch the foreskin opening, stopping immediately when resistance or discomfort occurs.
The retraction exercises should progress gradually over the treatment period, with patients typically achieving small increments of improvement each week. Initial sessions may only allow minimal movement of the foreskin, but persistent gentle stretching combined with the anti-inflammatory effects of betamethasone typically produces cumulative improvements. The exercises should never cause pain or force the foreskin beyond its natural limits , as excessive force can create scarring that worsens the condition. Parents performing these exercises on children require careful instruction to ensure appropriate technique and pressure application.
Frequency guidelines: twice-daily application schedule
The twice-daily application schedule for betamethasone in phimosis treatment represents an evidence-based regimen that balances therapeutic efficacy with safety considerations. Morning and evening applications, spaced approximately 12 hours apart, maintain optimal tissue drug concentrations whilst allowing for natural recovery periods between treatments. This schedule also fits conveniently into most patients’ daily routines, typically coinciding with morning and bedtime hygiene activities.
Consistency in timing proves more important than absolute precision, though significant deviations from the 12-hour interval should be avoided. If a dose is missed, patients should apply the cream as soon as remembered, unless it’s nearly time for the next scheduled application. Double-dosing to compensate for missed applications is contraindicated and may increase the risk of adverse effects without providing additional therapeutic benefit. The twice-daily schedule also allows for adequate monitoring of treatment response and early identification of any adverse reactions.
Duration parameters: 4-8 week treatment protocols
Treatment duration with betamethasone for phimosis typically ranges from 4-8 weeks, with most patients achieving satisfactory results within this timeframe. The initial 4-week period represents the minimum effective treatment duration, during which the majority of therapeutic improvement occurs. However, some patients, particularly those with severe phimosis or extensive scarring, may require extended treatment up to 8 weeks to achieve optimal outcomes.
Clinical response monitoring during the treatment period helps determine the optimal duration for individual patients. Significant improvement typically becomes apparent within the first 2-3 weeks , with continued progress throughout the treatment course. Patients who show no improvement after 4 weeks of consistent treatment may benefit from treatment extension, alternative therapy, or surgical consultation. The decision to extend treatment beyond 8 weeks requires careful consideration of risk-benefit ratios and specialist consultation to evaluate alternative treatment options.
Monitoring treatment progress and foreskin retractability assessment
Systematic monitoring of treatment progress represents a critical component of successful betamethasone therapy for phimosis, enabling healthcare providers to assess therapeutic efficacy and make appropriate treatment modifications when necessary. Progress evaluation should occur at regular intervals, typically at 2-week increments during the initial treatment phase, allowing for early identification of both positive responses and potential complications. This monitoring approach ensures that patients receive optimal care whilst minimising the duration of ineffective treatments.
Objective assessment methods include photographic documentation, measurement of retractable foreskin diameter, and standardised scoring systems that quantify the degree of phimotic severity. The Kikiros grading system, which categorises phimosis severity from Grade 0 (no phimosis) to Grade 5 (severe phimosis with complete non-retractability), provides a reliable framework for tracking improvement over time. Patients typically demonstrate improvement of at least one grade level within the first month of treatment , with continued progress throughout the therapeutic course.
Patient-reported outcomes also play a valuable role in progress monitoring, particularly regarding comfort levels during retraction attempts and any associated symptoms such as pain or irritation. Parents of paediatric patients should be trained to recognise signs of improvement, including increased foreskin mobility, reduced tissue thickness, and easier hygiene maintenance. Healthcare providers should establish clear communication channels to address concerns promptly and adjust treatment protocols based on individual patient responses and any emerging complications.
Managing side effects and treatment complications
Whilst betamethasone cream demonstrates an excellent safety profile for phimosis treatment, vigilant monitoring for potential side effects remains essential throughout the therapeutic course. The most commonly observed adverse effects include mild skin irritation, temporary burning sensations upon application, and occasional contact dermatitis in sensitive individuals. These side effects typically occur within the first week of treatment and often resolve spontaneously as the skin adapts to the medication.
More serious complications, though rare, can include skin atrophy, striae formation, and systemic corticosteroid effects, particularly with prolonged use or excessive application. The risk of significant adverse effects increases with treatment duration beyond recommended protocols , emphasising the importance of adhering to established treatment guidelines and regular monitoring schedules. Healthcare providers must educate patients and caregivers about recognising early signs of complications and establishing clear protocols for seeking immediate medical attention when concerning symptoms develop.
Skin atrophy recognition and prevention strategies
Skin atrophy represents one of the most significant potential complications of prolonged topical corticosteroid use, manifesting as thinning of the epidermis and dermis with increased fragility and susceptibility to injury. In the context of phimosis treatment, skin atrophy typically appears as a shiny, translucent appearance of the foreskin with increased visibility of underlying blood vessels. The affected tissue may feel thinner and more fragile than normal, with increased risk of tears during gentle manipulation.
Prevention strategies focus primarily on adherence to recommended dosing protocols and treatment durations, avoiding excessive cream application, and implementing regular monitoring schedules. The use of drug holidays, where treatment is temporarily discontinued for 1-2 weeks, can help prevent atrophy development whilst maintaining therapeutic benefits. If early signs of atrophy develop, treatment should be immediately discontinued, and the affected area should be monitored for resolution of atrophic changes. In most cases, mild atrophy resolves completely within several weeks of treatment discontinuation.
Allergic contact dermatitis identification
Allergic contact dermatitis to betamethasone or its excipients presents a significant treatment complication that requires immediate recognition and management. This condition typically manifests as erythema, swelling, vesicle formation, and intense itching that develops within hours to days of cream application. Unlike the mild irritation that some patients experience initially, allergic dermatitis tends to worsen with continued exposure and may spread beyond the application site.
Diagnostic confirmation often requires patch testing to identify specific allergens, though clinical presentation and temporal relationship to treatment initiation usually provide sufficient evidence for diagnosis. The incidence of allergic reactions to betamethasone remains relatively low, occurring in less than 2% of treated patients. Management involves immediate discontinuation of the offending agent, application of cool compresses, and potential use of oral antihistamines or alternative topical anti-inflammatory agents under medical supervision.
Systemic absorption risks in paediatric patients
Paediatric patients face increased risks of systemic corticosteroid absorption due to their higher surface area-to-body weight ratio and thinner skin barrier compared to adults. Systemic absorption can potentially lead to hypothalamic-pituitary-adrenal axis suppression, growth retardation, and other manifestations of systemic corticosteroid excess. These risks emphasise the importance of careful dosing, limited treatment areas, and appropriate
duration limitations when treating children.Monitoring for signs of systemic absorption includes assessment for cushingoid features, growth velocity changes, and behavioral alterations that may indicate corticosteroid excess. Healthcare providers should establish baseline height and weight measurements before initiating treatment, particularly for children requiring extended therapy courses. Parents should be educated about recognising early signs of systemic effects, including increased appetite, mood changes, and any unusual physical symptoms that develop during treatment.
Treatment failure scenarios and alternative therapeutic options
Despite betamethasone cream’s impressive success rates, approximately 5-35% of patients may not achieve satisfactory foreskin retractability within the standard treatment timeframe. Treatment failure typically becomes apparent when no significant improvement occurs after 4-6 weeks of consistent, properly applied therapy. Several factors contribute to treatment resistance, including severe fibrotic scarring, underlying skin conditions such as lichen sclerosus, and patient non-compliance with application protocols.
Alternative therapeutic approaches for betamethasone-resistant cases include switching to different topical corticosteroids such as clobetasol propionate 0.05%, which demonstrates higher potency than betamethasone. Some practitioners advocate for combination therapy incorporating topical calcineurin inhibitors like tacrolimus, particularly in cases where corticosteroid side effects limit treatment duration. Hyaluronidase injections have shown promise in preliminary studies, though this approach requires specialist administration and remains investigational in most clinical settings.
Surgical intervention becomes necessary when conservative treatments fail to achieve functional improvement after exhaustive medical management. Circumcision remains the definitive treatment option, though partial procedures such as dorsal slit or preputioplasty may preserve more foreskin tissue whilst addressing the mechanical obstruction. The decision to proceed with surgery requires careful consideration of patient age, severity of symptoms, and individual preferences regarding foreskin preservation versus complete resolution of the condition.
Post-treatment maintenance and long-term foreskin care
Successful completion of betamethasone therapy for phimosis requires ongoing maintenance strategies to preserve treatment gains and prevent recurrence of foreskin tightness. Post-treatment care protocols emphasise regular foreskin retraction during daily hygiene routines, ensuring that newly gained mobility is maintained through consistent, gentle manipulation. Patients should incorporate foreskin retraction into their normal bathing routine, gradually working up to complete retraction as tissue comfort allows.
Long-term hygiene practices play a crucial role in preventing recurrent phimosis and associated complications such as balanitis or smegma accumulation. Daily gentle cleansing with warm water and mild soap helps maintain optimal tissue health whilst preventing the inflammatory processes that can lead to renewed foreskin tightness. Patients should avoid harsh soaps, excessive scrubbing, or forceful retraction that might cause micro-trauma and subsequent scarring.
Monitoring for signs of recurrence should continue indefinitely, with patients educated about early warning signs that might indicate returning foreskin tightness. These signs include increased difficulty with retraction, development of white scarring bands, or recurrent episodes of balanoposthitis. Early recognition of these symptoms allows for prompt intervention with topical therapy, potentially preventing the need for more intensive treatments or surgical procedures.
Healthcare providers should establish follow-up schedules that include assessment at 3, 6, and 12 months post-treatment completion, with annual evaluations thereafter for pediatric patients until they reach adulthood. Adult patients may require less frequent monitoring but should maintain awareness of potential recurrence risks, particularly in the presence of predisposing factors such as diabetes, poor hygiene practices, or recurrent genital infections. The long-term success of betamethasone therapy depends heavily on patient education and commitment to ongoing foreskin care practices that support sustained treatment benefits.